Scratching the surface

Roger Marchant asks whether chemicals produced by bacteria and yeast could replace the environmentally hazardous surfactants found in many everyday products
The Biologist 64(2) p7
Surfactants are a group of chemicals that have a daily impact on our lives and yet we have little awareness of their importance, or their ubiquitous use. The word 'surfactant' was coined many decades ago as a contraction of 'surface active agent', and has become a common term for a wide range of compounds that display physicochemical properties at the interfaces between liquids, gases and surfaces.
The most obvious manifestations of these effects are the foaming of liquids at the interface with air, and the stabilisation of emulsions formed of oil and water. It is these effects that make surfactants important components of the enormous range of detergents and surface cleaners we use every day.
Similarly, the emulsification capabilities of surfactants are crucial for the maintenance of stable emulsions in many foodstuffs, and in cosmetic and pharmaceutical preparations. There are even applications for surfactants in products such as paints and inks, and they are now finding a role in environmental remediation, particularly where hydrocarbon contamination or heavy metals are involved.
The world market for surfactants is huge, with total annual usage estimated to be more than 13 million tonnes.
It may appear, then, that this is a mature technology with little pressure for immediate change. This is not the case for a number of reasons. First, many of the commonly used surfactants, such as LAS (linear alkylbenzene sulphonate) and SLES (sodium lauryl ether sulphate) are produced from non-renewable hydrocarbon or synthetic feedstocks. Second, the biodegradability of many of these synthetic surfactants in the environment is poor and they present a toxic hazard to aquatic organisms.
In the food industry, emulsifying and gelling agents often come from natural sources, such as soya lecithin and egg albumen, but even here there is some pressure to seek alternatives – either through the desire to make the final product suitable for vegans or because the manufacturer wants to avoid any genetically manipulated material in the product (soya lecithin from non-GM crops is now difficult to source, for example).
The question now being asked is whether there are any viable alternatives to many of the existing surfactants – both synthetic and natural. The answer to this question, based on ongoing research carried out in the last few decades, is yes: the solution lies in the production of biosurfactants.
Biosurfactants, quite simply, are surfactant molecules synthesised by living organisms. Some are produced by plants and animals, but by far the most extensively studied – and the ones with the greatest potential for exploitation – come from microorganisms.
The main producers are bacteria and yeasts, and the biosurfactants are secondary metabolites that often accumulate in large quantities as cultures grow. Usefully, most of the biosurfactants are exported from the microbial cells into the growth medium, making separation and purification much easier than if the compounds were retained within the cells.
Microbial biosurfactants can be broadly divided into two groups: low molecular weight and high molecular weight molecules. The low molecular weight biosurfactants comprise glycolipids and lipopeptides, and have clear hydrophilic (water loving) and lipophilic (oil loving) regions. The hydrophilic part is either the sugar or peptide region, while the lipophilic area comprises alkyl chains of varying length.
The exact structure of the molecules determines the physicochemical characteristics, and dictates whether a particular biosurfactant has good detergency and foaming characteristics or is a good emulsifier.
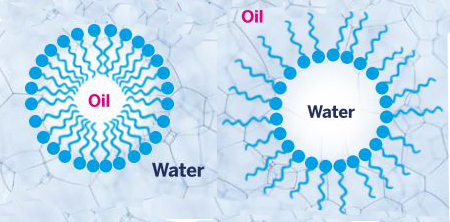 Fig. 1 Biosurfactants produce emulsions by forming micelles of oil in water or water in oil. The head of the biosurfactant is hydrophilic and the tail is lipophilic/hydrophobic.The property of foaming is simply another expression of the interface phenomenon – in this case, between an aqueous environment and a gas.
Fig. 1 Biosurfactants produce emulsions by forming micelles of oil in water or water in oil. The head of the biosurfactant is hydrophilic and the tail is lipophilic/hydrophobic.The property of foaming is simply another expression of the interface phenomenon – in this case, between an aqueous environment and a gas.The high molecular weight biosurfactants are generally good emulsifiers or gelling agents, and comprise lipopolysaccharides and lipoproteins. To date these large polymers have been less fully examined than the low molecular weight biosurfactants.
Why do microorganisms produce biosurfactants? This is not a simple question to answer, as it appears that they have a number of functions in the life of these organisms. One key function, particularly for organisms that use oily substrates for growth or degrade hydrocarbons, especially in the marine environment, is to make the substrate available for the microorganism to use. It is also clear that biosurfactants can play a role in the motility of bacteria and that their synthesis is closely linked to the quorum-sensing mechanisms of the cell, which alters gene expression based on the density of its surrounding cells.
Interestingly, there is evidence that for some biofilm-forming bacteria, the production of a biosurfactant molecule – which disrupts the integrity of the biofilm – can be important in sustaining channels for gas and nutrient exchange to the cells at the base of the biofilm.
While functions have been postulated for biosurfactants in a number of bacteria, the reason why a range of yeasts produce large quantities of biosurfactants is not yet clear.
While biosurfactants could provide a viable alternative to existing synthetic surfactants, we must ask what the drivers for and against such a switch are.
The main advantage of biosurfactants is that they can be produced from abundant, renewable feedstocks. In general, their production from simple sugar substrates is rather poor, and better yields are obtained when oily feedstocks such as oleic acid or rapeseed oil are used, but other agricultural oils would also be suitable. It has been suggested that they could be produced from waste materials such as used cooking oil.
Toxicology testing of a range of biosurfactants has shown that they present a very low risk to organisms in the environment and are readily biodegraded to completely harmless catabolites.
From a performance point of view, biosurfactants are active at very low concentrations, are not adversely affected by environmental conditions such as high salt levels and function over a wide temperature range. They have been shown to perform well as components in a number of consumer product formulations, particularly when used as a partial replacement for a synthetic surfactant.
Biosurfactants do have some disadvantages, though, and these need to be resolved before they can be used extensively in consumer products.
The main difficulty is connected with downstream processing – the methods that must be used to separate the pure biosurfactants once they have been produced by the microorganism. Since biosurfactants are probably never synthesised by a microorganism as a single molecular type, but as a mixture of very similar molecules, and since these molecules vary only slightly in their chemical structure and properties, separation of a pure product may be a key challenge.
Glycolipid biosurfactants seem to be amenable to separation and isolation into individual molecular types using established technology such as 'supercritical CO2', which is cheap to carry out. This method is already employed in the food industry – for example, in the production of dried coffee products – and relies on pressure and liquefied CO2 rather than organic solvents.
However, the most expensive part of the biosurfactant production process is the energy required for the fermentation step. Therefore, it will be imperative to use a microbial strain that gives high yields in short fermentation runs. The final cost of the product will be the determining factor in whether biosurfactants become common components of consumer products.
Potential candidates for biosurfactant production
One of the first organisms to be investigated for commercial biosurfactant production was the bacterium Pseudomonas aeruginosa, which produces a set of glycolipid biosurfactants known as rhamnolipids.
The rhamnolipid is produced by the organism as it enters the stationary phase of growth, and seems to serve a range of functions for the bacterium, including maintenance of biofilm structure and motility. Although the rhamnolipids produced by P. aeruginosa are highly effective as detergents, manufacturers have been reluctant to consider them as the organism is classified as a type II opportunistic pathogen.
Probably because of the kind of function the rhamnolipids carry out, their synthesis is highly regulated by P. aeruginosa's quorum-sensing system, making overproduction difficult to achieve.
Alternative non-pathogenic producers of rhamnolipids are currently under investigation – for example, Burkholderia thailandensis. This may provide a route to commercialisation, but perhaps the best prospects for biosurfactants lie with the yeast producers, which are non-pathogenic and appear not to have the same systems regulating expression as some bacteria.
Starmerella bombicola produces a glycolipid biosurfactant, sophorolipid, which can be produced in large-scale fermentations with yields of at least 100g/l. Again, the organism does not produce a single molecular type, but a range of both acidic and lactonic forms. It has recently been shown that sophorolipids have some potentially valuable additional biological properties such as disruptors of bacterial biofilms (Figure 2, below), bactericidal and bacteriostatic activity, and selective killing activity against human cancer cell lines.
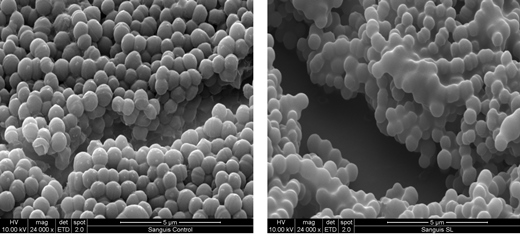
Figure 2 Scanning electron micrographs of biofilm in the oral bacterium Streptococcus sanguinis before (left) and after (right) treatment with 1% lactonic sophorolipid for 30 minutes. Spaces have appeared in the treated biofilm and the appearance of the cell surfaces has been modified. Bacteria may release surfactants to create channels for nutrients into the colony. (Images kindly provided by Mohamed Elshikh)
The critical observation that has been made is that the acidic and lactonic forms display very different biological activity, which demands great care in the execution of any experimental work on bioactivity.
There are other biosurfactants that are currently under investigation – for example, mannosylerythritol lipids produced by the yeast Pseudozyma aphidis, and trehalolipids, cellobiose lipids and subtilisin (a lipopeptide), which are produced by the bacterium Bacillus subtilis.
Leading the way
There are a few consumer products around the world that already contain microbial biosurfactants, principally if not exclusively sophorolipids. These include cleaning and detergent products in Europe and the Far East, and also some over-the-counter pharmaceuticals in the Far East.
Would we notice if microbial biosurfactants started to appear in our domestic products? Probably not, unless the manufacturer chose to make a specific point in relation to the green credentials of their product or wished to target their product to specific sectors such as the anti-GM or vegan food markets.
The achievement of good production yields of certain biosurfactants at a competitive market price (around £2/kg) could lead to microbial biosurfactants becoming a key component of many products in our cupboards and larders.
A measure of the current commercial interest in biosurfactants is the number and diversity of European Union-funded research projects that involve biosurfactants – for example, the FP7 KillSpill project, which examines the remediation of marine oil spills; the FP7 Biosurfing project, which focuses on the production of 'new to nature' sophorolipids with applications in the food and pharmaceutical sectors; and the Horizon 2020 Marisurf project, which is pioneering the search for new biosurfactants from marine bacteria for a range of potential applications.
Roger Marchant is professor of microbial biotechnology at the School of Biomedical Sciences, Ulster University.
1) Marchant, R. & Banat, I. M. Microbial biosurfactants: challenges and opportunities for future exploitation. Trends in Biotechnology 30, 558–565 (2012).
2) Franzetti, A. et al. Biosurfactant use in heavy metal removal from industrial effluents and contaminated sites. In Biosurfactants: Production and Utilization – Processes, Technologies and Economics (eds Kosaric, N. & Sukan, F. V.) 361–369 (CRC Press, 2014).
3) Díaz de Rienzo, M. et al. Antibacterial properties of biosurfactants against selected Gram-positive and negative bacteria. FEMS Microbiology Letters 363, 224–231 (2016).
4) Elshikh, M. et al. Biosurfactants: Promising bioactive molecules for oral-related health applications. FEMS Microbiology Letters 363(18) (e-Published 2016).


