War of the Worms
Parasitic worms blight the lives of billions of people worldwide. Stefano Colombo looks at the challenges of vaccinating against them
The Biologist 64(1) p10-13
The idea of human bodies being hijacked by parasites might seem like something out of a science-fiction novel. However, to much of the developing world, parasitic infection is a daily reality, with severe consequences.
Parasitic worms – collectively known as helminths – are among the most common infectors of humans and livestock. Thanks to improvements in sanitation and healthcare, helminth infection is now rare in most first world countries. Globally, however, close to two billion people are estimated to host at least one type of worm, with many people infected with multiple species.
However, because helminth infection rarely results in death, it receives far less attention than other diseases with higher mortality rates and it is still considered a neglected tropical disease (NTD) by the World Health Organization.
However, that is not to say that their presence goes unfelt. Depending on the species of helminth, a range of symptoms can occur. Some are minor such as nausea and pneumonia caused by the larval stages of the hookworms Necator americanus and Ancylostoma duodenale. Others are more severe.
Children chronically infected with the whipworm Trichuris trichiura often suffer from cognitive deficits and growth stunting. The tissue-dwelling Onchocerca volvulus, transmitted by the black fly, can cause blindness when their larvae migrate through, and die within, the eyes. Perhaps the most extreme pathology is seen in those suffering from lymphatic filariasis, caused by three different species of helminth, where invasion of the lymphatic system results in extreme swelling of the limbs, known as elephantiasis, leaving the host permanently disfigured.
The impact of these parasites cannot be measured in lives lost, but rather in the amount of healthy life lost. Living with any of these symptoms creates a barrier to education and work, decreasing the infected person's quality of life. Many experiencing more severe signs of disease, such as swollen limbs or blindness, are stigmatised and ostracised within their communities. The combined burden of the symptoms mentioned above, and many more besides, holds back communities, preventing their development and trapping them in poverty. Although it is possible to develop immunity to helminth infection, it is rarely complete, so people living in areas endemic for these parasites will likely be infected throughout their lives. What is more, due to the slow development of resistance to infection, children suffer the highest burden of disease.
There are effective drug treatments to combat infection. Most antihelminthics are able to clear infection with few side-effects, but they have other drawbacks. First, they do not confer any protection to subsequent infections, with reinfection common after just six months, making regular treatment essential. Organising repeated treatments in countries with poor infrastructure and little money is challenging, and de-worming campaigns are often discontinued due to political or economic instability. Past efforts have proved unsuccessful at eradicating targeted parasites.
Another issue is the development of drug resistance. Widespread use of antihelminthics in livestock has resulted in the rapid development of drug-resistant strains of livestock-specific species. While evidence of resistant helminths in humans remains anecdotal, we can see from other infections such as malaria and Staphylococcus that such a challenge is likely on the horizon.
The gold standard for controlling disease is vaccination. By exposing an individual to a harmless version of a pathogen, vaccines mimic infection and prime the immune system to defend the body without regular drug treatment. Vaccines provide long-term protection from infection and are less vulnerable to the emergence of parasite resistance.
However, developing vaccines is no easy task, and helminths provide some unique challenges to the development process. A vaccine against a virus or bacterium may be comprised of a live version of that bug that doesn't cause disease, or a completely inactive version. This isn't possible with helminths, as their life cycle cannot be completed outside of their host (that is, human beings), making it impossible to breed and mass produce such a non-pathogenic or inactive parasite.
Another way to create a vaccine is to produce materials derived from the pathogen, known as antigens, that elicit an immune response in the host. However, while this is definitely a more viable way to create anti-helminth vaccines, it is not without its own difficulties. Helminths are complex, multi-cellular organisms comprised of myriad different molecular components, which must be sorted through when identifying candidate antigens. Researchers are looking for the proverbial needle in a haystack.
When searching for the right material for a vaccine, there are some key features that go into the selection process. A good target antigen may be a protein produced by the parasite essential to its survival – for example, an enzyme secreted by the larvae that allows them to migrate through their host.
The antigen should also be easily accessed by the immune system – for example, immune cells would never be able to interact with a protein found only in cells deep inside the parasite. This means molecules either found on the surface of the parasite or secreted by it are the most promising. The material you select must also be suitable for mass production by modern biotechnological methods.
Hookworms begin life as microscopic eggs passed in the faeces of an infected host. They hatch and mature in soil, where they live as small larvae, roughly 600µm in length.
When they encounter a potential host, they penetrate the skin into the vasculature and circulate until they reach the pulmonary vessels, where they penetrate the walls of the alveoli and migrate through the lungs.
Once at the top of the airways, they drop down the oesophagus and make their way to the small intestine. Here, they grow by moulting until they are finally sexually mature adults.
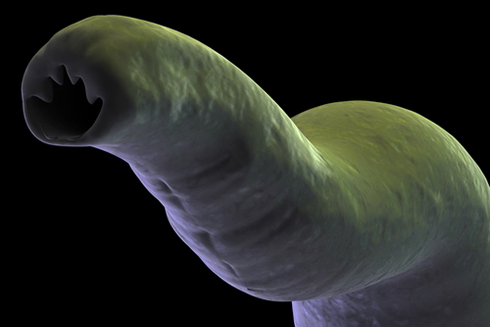
Mature hookworms vary from 5 to 13mm in length. They have narrow, translucent bodies comprised of little more than a digestive tract and reproductive organs.
Their most distinctive feature is their mouth, which contains vampire-like teeth – known as cutting plates – that enable the parasite to attach itself to the intestinal wall and feed off the host's blood.
Unfortunately, helminths make identifying antigenic material difficult. They have evolved to be master manipulators of the immune system, spending much of their life cycle under the radar not inducing an immune reaction. It is thought that some helminths can secrete chemicals that down-regulate the elements of the immune system involved in expelling them. This immunosuppressive quality is so potent that low-level infection with certain helminths is being trialled as a treatment for aggressive inflammatory diseases such as inflammatory bowel disease.
When the body does generate protective immunity against a helminth, the type of response generated is known as a 'type-2' response. This is characterised by the production of certain molecular signalling molecules, known as cytokines, such as interleukin (IL)-4, IL-5 and IL-13, as well as the influx of eosinophils, the differentiation of type-2 T helper cells and the production of immunoglobulin E.
This natural response is likely to have evolved to deal with infection by multicellular parasites. An excessive, inappropriate, type-2 response against a harmless molecule is known as an allergic reaction.
This means antigens for anti-helminth vaccines must be selected carefully, as those that generate too potent a response risk inducing a dangerous allergy in patients. A previous vaccine trial for the hookworm N. americanus was halted after some volunteers experienced rashes across their head and torso linked to type-2 allergic inflammation.
Other hurdles faced by researchers are more practical than biological. As helminths are primarily endemic to the developing world, access to patients and samples can prove challenging. Good scientific practice requires a sterile environment and a range of expensive equipment, making fieldwork a tricky and costly business. Unfortunately, it seems testing these vaccines on willing subjects in the developed world has its own problems.
The trialled hookworm vaccine mentioned above was first tested in the US on subjects who had not previously encountered infection. This group experienced no adverse effects from the vaccine. This suggests that a person's history of exposure to infection plays a critical role in determining how they will respond to vaccination. As such, trials must be conducted in endemic regions.
Creating a novel vaccine requires as complete an understanding as possible of how the immune system reacts to infection. Unfortunately, acquiring such an understanding is difficult, as studies in humans are limited by ethical and practical considerations. Travelling to endemic regions to find study cohorts is challenging enough and, once there, researchers are typically restricted to blood and stool samples, which only provide a small window into the complexities of immunity. Furthermore, results from the field are often confounded by variables outside the study's control, such as previous infection history, prior treatment with antihelminthics, co-infection with other diseases such as malaria, and differing levels of parasite exposure.
An alternative to field studies is the use of laboratory models. This involves studying species of helminth that infect rodents and drawing parallels between them and human infection. These have been invaluable in providing new insights into anti-helminth immunity. Model parasites usually mirror the life cycle of their human counterparts and appear to interact with the immune system in similar ways.
However, humans and rodents are physiologically very different and what applies in mice does not always apply in humans.
Additionally, laboratory models do not often reflect infection in the field. Under experimental conditions, rodents are typically treated with a large single dose of a given parasite. People, on the other hand, experience repeated exposure to small numbers of many different species of parasite. Refining our model systems to better reflect the infection status seen in humans will be a crucial step in expanding our understanding of the immunology behind helminth infection.
However, perhaps the most substantial roadblock to the development of anti-helminth vaccines is the lack of commercial interest in their discovery.
Those affected have little money to spend on medicine, and with medical research costs being astronomical, commercial vaccine development is not viable. As such, vaccine development against helminths and other NTDs is a charitable pursuit by academic labs and government-driven medical enterprise.
However, policymakers in both endemic countries and in the developed world often have limited knowledge of these diseases, meaning raising awareness is crucial to ensure ongoing research.
The Sabin Vaccine Institute, a non-profit organisation dedicated to the fight against NTDs, is currently trialling two novel anti-helminth vaccines.
One vaccine targets two enzymes – NaGST-1 and NaAPR-1 – thought to be essential for hookworm to digest haemoglobin. Phase I trials have recently concluded in Brazil, Gabon and Washington.
In addition, a phase I trial for an anti-schistosomiasis (bilharzia) vaccine is underway in Texas. Both have shown promise in animal models.
Stefano Colombo AMRSB is a Wellcome Trust PhD student at The University of Manchester. His research focuses on the immune response to gastrointestinal helminth infection.


