Frozen assets

Advances in the science of ‘cryobanking’ animal gametes are helping restore endangered populations and preserve biodiversity, writes William V Holt
The Biologist 64(4) p16-21
In the late 1940s a young postdoc called Chris Polge, working at the MRC laboratories in Mill Hill, north London, was tasked with working out how to preserve sperm by freeze-thawing. He made the fortuitous but accidental discovery that mammalian and fowl sperm could be frozen and thawed successfully in the presence of glycerol[1].
After many unsuccessful attempts to freeze sperm in various sugar solutions, all his stock solutions were left unattended in a fridge for some months while the project was in abeyance. The labels duly fell off his bottles of solutions, but when this project was resumed, he and his colleagues put the labels back as best they could.
Unknowingly, a bottle of histological slide-mounting fluid containing glycerol became mislabelled and therefore was used in the next experiment. It must have been a eureka moment to find that one of the ‘sugar’ solutions successfully cryopreserved the sperm! But after failing to repeat the experiment with conventional solutions, the mislabelled bottle was eventually analysed and the presence of glycerol was uncovered.
The fortunate discovery led to the large-scale application of semen cryopreservation in cattle breeding and the complete transformation of the global dairy industry, which nowadays relies almost entirely on the use of frozen semen and artificial insemination for cattle breeding.
Similar semen freezing methods based on the cryoprotective effects of glycerol are also widely used in human fertility clinics for the preservation of semen. Polge went on to become director of the Animal Research Station in Cambridge and made further significant contributions to reproductive technologies for domestic animals.
Semen freezing has also been used successfully to preserve fertile sperm from numerous wild mammals, fishes, amphibians and birds. The realisation that, in principle, it is feasible to collect sperm from diverse species and keep them in liquid nitrogen (-196ºC) has led several research groups to envisage the systematic collection and storage of semen from many of the species heading towards extinction[2,3].
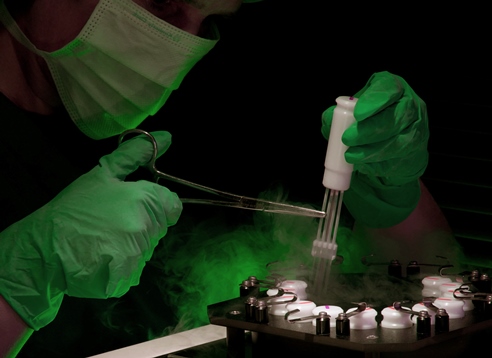 A researcher ‘seeding’ samples in cryo straws (image courtesy of Planer PLC).
A researcher ‘seeding’ samples in cryo straws (image courtesy of Planer PLC). These collections of frozen semen are widely regarded as ‘gene banks’ or ‘genome resource banks (GRBs)’ and, in the conservation context, the general aim is to use the frozen semen within planned breeding programmes to counter the deleterious effects of inbreeding.
Computer simulations[4] show that judicious semen sampling from genetically important males combined with the insemination of genetically important females could, in principle, delay or prevent the extinction of some threatened species. Unfortunately, differences between species are hugely influential in determining the success of semen cryopreservation protocols[5], and it is still not possible, even after seven decades of semen cryopreservation technology, to predict the best technical approaches.
We know that frozen-thawed semen has a shorter survival time and worse functionality than fresh semen, effects that are crucially important in the context of artificial insemination. Here it is essential that the sperm stay alive long enough to be around when the female ovulates. This is an important general principle in any type of assisted reproductive technology, whether in mammals, fishes, reptiles, amphibians or birds.
Logically, it means that anyone contemplating an artificial insemination project has to know as precisely as possible when to carry out an insemination.
Thus a bank of frozen semen is only useful for breeding purposes if it is supported by considerable knowledge of the reproductive biology of females. This is frequently a problem for scientists working with wild but threatened species given that they are probably faced with the task of discovering these important reproductive characteristics of female physiology for themselves.
At this point it is essential to distinguish between those GRBs that focus only on collections of DNA, blood and/or tissues of various types, and those that focus on reproductive cells, particularly sperm, oocytes, embryos and stem cells. The former type of GRB represents a major scientific resource and millions of samples are maintained worldwide for scientific research in many well-organised collections (see, for example, the iDigBio in the US and The Frozen Ark, a UK-based international consortium of biobanks).
 Frozen sperm of the endangered Wyoming toad was used to produce 100,000 offspring for release into the wild (image courtesy of Sara Armstrong)
Frozen sperm of the endangered Wyoming toad was used to produce 100,000 offspring for release into the wild (image courtesy of Sara Armstrong)Such collections are valuable for studying many aspects of evolutionary biology and genetics, especially as the species represented may be on the brink of extinction and therefore no longer available for study.
However, reproductive GRBs contain genetic resources that are potentially useful for supporting the wellbeing and survival of populations by combating inbreeding. If semen and other reproductive cells are preserved for the long term, it means that the individuals they represent are still part of the breeding population, even years after their death[6].
In theory, at least, frozen sperm samples remain viable and fertile for many years and can be used to reinvigorate the genetic diversity of inbred populations in years to come. This type of GRB is the animal equivalent of Kew’s Millennium Seed Bank and the Svalbard Global Seed Vault, which aim to preserve the world’s plant genetic resources for the future in ways that retain their viability.
Success stories
The practical use of reproductive GRBs relies on technologies of varying and unpredictable degrees of sophistication. For species with domesticated relatives – such as wild cattle – the establishment of artificial insemination techniques, and possibly even embryo transfer methodologies, is reasonably straightforward.
However, for other species the required technologies may be unexpectedly complex. For example, the successful establishment of a GRB and associated reproductive technologies for giant panda (Ailuropoda melanoleuca) conservation in China required a great deal of investment in understanding female social behaviour and detailed knowledge of endocrinology.
Despite its complexity the project is enjoying so much success in China that the giant panda’s threat status has been downlisted by the IUCN from endangered to vulnerable. Likewise, the recovery from near extinction of the black-footed ferret (Mustela nigripes) in the US was accomplished by a combination of reproductive technologies, including semen freezing and artificial insemination, through a programme that began in the 1980s and has restored more than 4,000 individuals to 20 sites across eight US states, Canada and Mexico[7].
Samples of frozen semen from the original founder animals are still used judiciously to support the extant population and genetic analyses have clearly shown that the kits born following artificial insemination are much less inbred than those born after natural mating.
Although the principle behind the establishment of reproductive GRBs as species-support tools is sound, it is not a trivial undertaking. Many major decisions, some painful, have to be made because a useful GRB requires long-term investment and achievable aims.
It is salutary to note that, although attempts to freeze semen have been recorded in about 100 different mammalian species[5], only the giant panda and black-footed ferret projects can be considered as serious examples of GRB success.
Mammals are not necessarily the only group of species that could be supported using banks of frozen sperm. One of the first fish-based GRBs was established in 1981 at the National Academy of Science of Ukraine in the former USSR. The genetic resources preserved in this cryobank are of unique importance. The bank contains frozen sperm from an impressive range of fishes, primarily from carp, sturgeon and salmonid species collected from the different republics of the former USSR.
Many of the samples are from species listed as close to extinction, including the ship sturgeon (Acipenser nudiventris), formerly abundant in the Black, Aral and Caspian seas, but now listed as critically endangered; the Russian sturgeon (Acipenser gueldenstaedtii), also critically endangered; and the green sturgeon (Acipenser medirostris), which is native to the Pacific Ocean. It is widely acknowledged that this cryobank, which is maintained on a very limited budget, represents a unique resource for future breeding or restoration programmes for endangered species.
As many species of fish reproduce by external fertilisation, and also as fish sperm generally respond well to cryopreservation methods, having frozen sperm stocks means that if females can be induced to spawn fresh eggs it is technically possible to generate embryos.
 The establishment of a specialist gamete resource bank, alongside other reproductive technologies, have helped restablish giant panda numbers in China. They have since been reclassified from endangered to vulnerable.
The establishment of a specialist gamete resource bank, alongside other reproductive technologies, have helped restablish giant panda numbers in China. They have since been reclassified from endangered to vulnerable.Save the toads and frogs
The fungal disease chytridiomycosis – which, together with ranaviruses, is currently causing havoc and extinctions among the world’s amphibian populations – has stimulated a great deal of interest in the possibility of using cryobanking to ensure the survival of threatened amphibians.
It is estimated that about 950 amphibian species are so endangered that they should be taken into biosecure facilities to ensure their survival. However, in reality the world’s zoos and conservation centres do not have enough space and resources to accommodate this many species.
This has stimulated the establishment of GRBs for frogs and toads within several countries around the world, especially the US, Australia and some South American countries. Female amphibians can be induced to produce eggs if injected with a hormone such as human chorionic gonadotropin and they can be fertilised externally by the frozen/thawed sperm. In this instance, fertilisation takes place in water, avoiding the complexities of trying to reproduce the conditions of the mammalian or avian oviducts.
Such methods have already been used by a research group at Memphis Zoo in the US, which aimed to breed endangered Wyoming toads (Anaxyrus baxteri) in captivity[8]. These methods led to the production and release of more than 100,000 offspring into the wild.
Interestingly, through a quirk of amphibian anatomy, sperm are ejaculated via the urinary bladder. A number of researchers have shown that if males are induced to urinate, they produce urine that is loaded with sperm that are still fertile after the spermic urine has been frozen and thawed. This was a key advance for non-invasive sperm collection and means that sperm can be collected more than once from the same animal.
The development of GRBs is not restricted to vertebrates either. It is worth noting that there has been a major initiative, led by Dr Mary Hagedorn of the Smithsonian Institution in the US, aimed at cryopreserving coral larvae so that they can be used for the restoration of reefs. There are three main sites in the world where coral cells are stored long term: at the Smithsonian Institution at the Hawaii Institute of Marine Biology, at the US Department of Agriculture’s National Animal Germplasm Program and at the Taronga Western Plains Zoo, Australia.
Gaps in progress
In case the text above has given a falsely optimistic view of sperm cryopreservation, it is worth pointing out that to date there has not been any success with artificial insemination using cryopreserved semen in marsupials. Only the koala (Phascolarctos cinereus) has been bred successfully and reliably by artificial insemination, and this was by the use of semen that was stored at 5°C, not frozen[9].
The complete failure of sperm cryopreservation efforts for marsupials has led some to propose that it is still worthwhile freezing the semen from those species that are under threat in the expectation that it will eventually be possible to use the apparently dysfunctional frozen-thawed sperm using a novel but as yet unknown method.
This hopeful approach is not without merit. Twenty years ago there was a need to develop technologies for the storage of mouse sperm in the context of biomedical science. This was not a trivial undertaking and, although the frozen-thawed mouse sperm could be used for in vitro fertilisation, they were not sufficiently functional for the rigours of artificial insemination. Today there are major international facilities devoted to the cryostorage of mouse sperm from numerous biomedically important strains. When required, these frozen sperm are thawed and used either by in vitro fertilisation or intracytoplasmic sperm injection for regenerating the particular strain of interest.
A tough egg to crack
While the relatively straightforward success of freezing fish and amphibian sperm has permitted the serious development of sperm-based GRBs for these species, the same cannot be said for their oocytes.For those species, many of which employ an external fertilisation strategy, oocytes are large cells that are typically encased in a tough and impermeable protective outer coating. This outer coat, known as the vitelline envelope, prevents the influx of all cryoprotectants and has resisted all attempts to develop preservation methods. Consequently, there is no successful way to preserve fish or amphibian oocytes, and researchers have been forced to investigate alternative techniques.
Solving this problem has required some innovative thinking and it is to the credit of several research groups that they refocused their approaches, exploring the possibility that various types of germ cells could be extracted from either ovaries or testes, frozen and stored, and then used to repopulate the ovaries and testes of recipient animals.
Such methods have proved remarkably successful. When cryopreserved spermatogonia extracted from the testes of rainbow trout (Oncorhynchus mykiss) were injected into the peritoneal cavity of recently hatched masu salmon (Oncorhynchus masou), they colonised the gonads of the recipients and were able to generate either spermatozoa or oocytes.
The procedures were successful using spermatogonia derived from dead fishes that had been in cold storage for five years[10]. These processes have also been used to help conserve a highly endangered fish species, the Manchurian trout (Brachymystax lenok), and seem to be widely applicable across fish species including catfish (Rhamdia quelen), brown trout (Salmo trutta m. fario) and the European grayling (Thymallus thymallus). As yet these successes have not been replicated in amphibians, but this may only be a matter of time and investment.
Setting up a cryobank
The establishment of reproductive cryobanks represents an open-ended financial and logistical commitment, and is therefore not to be undertaken lightly. It also involves complying with aspects of international law such as the Nagoya Protocol, which is especially relevant in this context as it is designed to protect against the unjust exploitation of a nation’s biodiversity and traditional knowledge without that nation receiving any benefit. Scenarios such as the development and patenting of novel medicines from endemic plants and animals have occurred in the past, but would now be illegal.
Most DNA and cell-based repositories keep their samples in -80°C freezers, but frozen collections of sperm, oocytes and embryos tend to use liquid nitrogen freezers that run at -196°C. Samples are maintained inside a variety of containers, including 0.25ml and 0.5ml plastic straws that are sealed at both ends, or screw-top cryotubes. Sample identification is vital and, although the containers are both labelled and colour-coded, precautions must be taken to avoid misidentifying and losing them.
 Storage dewars at Planer, a specialist in controlled temperature products.
Storage dewars at Planer, a specialist in controlled temperature products.Keeping track of samples through good database management is clearly essential and, given that many samples are probably from wild species, unambiguous genetic identification is crucial. It is not sufficient, for example, to classify a semen sample as having been obtained from an animal such as a Dorcas gazelle (Gazella dorcas); there are at least six subspecies whose coat colourings differ depending on their geographical origin, and future conservationists would want to match the origins of males and females if possible. Knowing this level of detail is extremely important; moreover, as phylogenetic classifications are sometimes revised in the light of new knowledge, the information systems have to be kept up to date.
Breakage of sample containers within liquid nitrogen storage is rare, but has to be treated seriously if it occurs. If contents are lost into the liquid nitrogen phase, bacteria and viruses are likely to be released into the liquid nitrogen itself, with the possibility of cross-contaminating other samples. This problem has been recognised relatively recently and there is a growing trend against immersing samples into the liquid phase, keeping the sample tubes instead in the dry, vapour phase of cold nitrogen. Biosecurity has to be a priority for all GRBs, especially as liquid nitrogen is usually not sterile – in fact, immersion in liquid nitrogen is an excellent way to preserve microorganisms.
Organisations aiming to maintain banks of viable samples have to guard against the risk that storage containers might fail unexpectedly, or of damage by flood or fire. Keeping duplicate samples offsite is a sensible precaution but is an open-ended expense. San Diego Zoo’s ‘frozen zoo’, which may be regarded as the world’s largest collection of wildlife-derived viable cells, sperm and embryos, is one institution that has taken this risk seriously and holds a duplicate collection offsite.
Fulfilling the technical, legal and organisational challenges of maintaining reproductive GRBs is an attractive, albeit expensive, prospect. Nevertheless, some biodiversity-rich countries recognise the potential conservation benefits.
The Indian Government has established the Laboratory for the Conservation of Endangered Species in Hyderabad, which is actively involved in the preservation of gametes and gonads, as well as DNA, live cells and tissue samples from local wildlife species. This organisation, the first of its kind in India, is aware of the need to link its gamete collection activities with the development of suitable reproductive technologies, and researchers have successfully produced a blackbuck (Antilope cervicapra) using frozen semen and artificial insemination.
Future prospects
Just as the development of cryopreservation technologies suddenly opened up possibilities for the cryopreservation and banking of viable sperm samples, researchers are now engaged in the search for alternative ways to preserve viable genomes for animal breeding. Recent novel approaches include drying and the long-term storage of nuclei at room temperature. These are ideal options for wild species in parts of the world that do not have access to liquid nitrogen or sustainable electric power for freezers.
The potential use of cultured viable cells for regenerating individuals by cloning is another technology that will undoubtedly be exploited in the future once the problems of producing abnormal and unfit offspring have been overcome. In fact, as the feasibility of amphibian cloning was first demonstrated in the 1960s by Sir John Gurdon[11] and his colleagues in Cambridge, it is time to revisit this technology with a focus on endangered species.
William V Holt FRSB is a professor of reproductive science at the Academic Unit of Reproductive and Developmental Medicine at the University of Sheffield and honorary research associate at the Smithsonian Conservation Biology Institute’s Center for Species Survival.
1) Polge, C. In Deep Freezing of Boar Semen (eds L. A. Johnson & K. Larrsson,), 167–173 (Swedish University of Agricultural Sciences, 1985).
2) Wildt, D. E. et al. Linkage of reproductive sciences: from ‘quick fix’ to ‘integrated’ conservation. J. Reprod. Fertil. Suppl. 57, 295–307 (2001).
3) Holt, W. V. et al. In Reproductive Science and Integrated Conservation 8 (eds Holt, W. V., Pickard, A. R., Rodger, J. C. & Wildt, D. E.), 267–280. (Cambridge University Press, 2003).
4) Harnal, V. K. et al. Computer simulations to determine the efficacy of different genome resource banking strategies for maintaining genetic diversity. Cryobiology 44, 122–131 (2002).
5) Charlton, S. J. et al. Strong heterogeneity in advances in cryopreservation techniques in the mammalian orders. Zoological Science 35, 1–22 (2018).
6) Holt, W. V. et al. Genetic resource banks in wildlife conservation. Journal of Zoology 238, 531–544 (1996).
7) Santymire, R. M. et al. In Reproductive Sciences in Animal Conservation (eds W. V. Holt , J. L. Brown, & P. Comizzoli), 119–134 (Springer, 2014).
8) Clulow, J. et al. Amphibian declines in the twenty-first century: why we need assisted reproductive technologies. Adv. Exp. Med. Biol. 753, 275–316, (2014).
9) Allen, C. D. et al. Successful artificial insemination in the koala (Phascolarctos cinereus) using extended and extended-chilled semen collected by electroejaculation. Biol. Reprod. 78, 661–666, (2008).
10) Lee, S. et al. Long-term (5 years) cryopreserved spermatogonia have high capacity to generate functional gametes via interspecies transplantation in salmonids. Cryobiology 73, 286–290 (2016).
11) Gurdon, J. B. & Hopwood, N. The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 44 (2000).
Bennet, J. Operating a Successful Cryobanking Facility. Planer, 2018.


