Beating animal coronaviruses
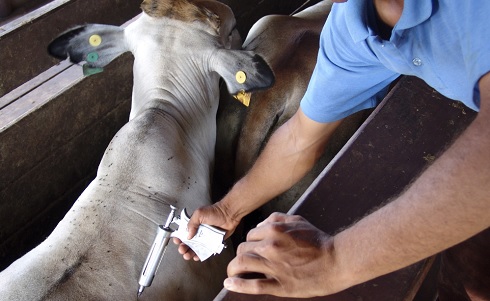
In veterinary medicine, vaccines have been successfully deployed to fight coronaviruses in pets and livestock for many years. Dr Michael James Francis explores what these vaccines can tell us about the likely success of a COVID-19 vaccination programme
December 4th 2020
The COVID-19 pandemic has reminded us all of the threat to humanity posed by zoonotic diseases. Indeed 60% of all infectious diseases affecting humans fall into this category and 75% of new human infections emerging in the last three decades are of animal origin[1]. This highlights the importance of a 'One Health' approach to disease control: the wellbeing of humans is inexorably linked to the health of animals and the environment in which we live[2].
With this in mind it is important that we should learn any lessons we can for COVID-19 disease control from existing coronaviruses that infect animals, and in particular those for which successful veterinary vaccines have already been developed. In addition to potential wildlife reservoirs of coronavirus infection (bats, civets, pangolins, mice, rats, etc.) there are a number of animal coronaviruses that infect livestock (cattle, swine, poultry, camels) and companion animals (dogs, cats).
These viruses cause a range of diseases that predominately result in respiratory symptoms or gastroenteritis. Successful commercial vaccines have been developed for the majority of these veterinary coronavirus diseases and there are undoubtedly some valuable observations for COVID-19 from such vaccination successes – and also from animal diseases that have proved to be more problematic.
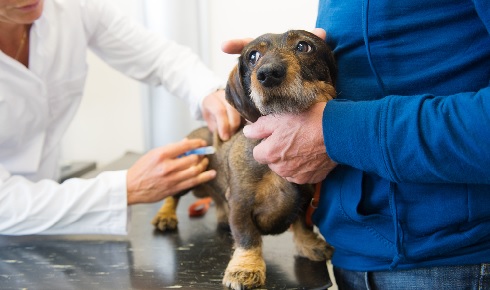 Puppies vaccinated against canine coronavirus require a second booster to maintain immunity beyond a year.
Puppies vaccinated against canine coronavirus require a second booster to maintain immunity beyond a year. The most important lesson that we should take away from veterinary medicine in our search for a vaccine against the SARS-CoV-2 virus is that both killed/inactivated vaccines and live/attenuated whole virus strategies have been successfully employed in the past to develop commercial coronavirus vaccines.
Furthermore, the development of vaccines against bovine coronavirus (BCV) and porcine transmissible gastroenteritis illustrates that passively acquired virus neutralising antibodies, derived through the transfer of maternal antibodies from vaccinated mothers to their newborns, play a major role in protective immunity[3]. This protection will persist as long as offspring are fed stored colostrum or allowed to naturally suckle from their mother. In addition, anti-BCV antibodies have been shown to be principally directed to the surface spike protein of the coronavirus and it is interesting to note that this protein is the target for the majority of the current leading vaccines against COVID-19.
A major concern for successful COVID-19 vaccination development has been speculation regarding the potential duration of immunity. This is often difficult to measure within human vaccine trials due to the lack of a controlled viral challenge. However, when developing veterinary vaccines it is generally necessary to conduct a virulent challenge of immunity to establish both the onset and duration of the protective immune response.
We know that the inactivated virus vaccines designed to combat canine coronavirus will generally elicit protective immunity 21 days after the primary vaccination course and, while the antibody levels will decline over the coming months, protection will last for at least one year. Following initial vaccination, an annual booster inoculation will maintain sufficient levels of immunity for a further year. However, it has proved difficult to induce prolonged strong immunity with some other animal coronavirus vaccines and producers often find it necessary to give multiple boosters at frequent intervals to maintain protection.
Nevertheless, it is reasonable to suggest that a one-year duration of immunity should be a minimal target for future COVID-19 vaccines and that through the use of novel vaccine technologies longer durations of immunity should potentially be achievable.
 Lessons from animal coronaviruses may be help public health officials broaden immunity offered by a vaccine if distinct or seasonal strains of COVID-19 emerge.
Lessons from animal coronaviruses may be help public health officials broaden immunity offered by a vaccine if distinct or seasonal strains of COVID-19 emerge. Cell-mediated immunity
While virus-neutralising antibodies directed against the coronavirus spike protein are critical in providing protective immunity following vaccination, a role for cell-mediated immunity cannot be ruled out. Indeed, it has been shown that cytotoxic T lymphocytes are responsible for early control of avian infectious bronchitis virus infection (IB) in poultry and that the transfer of T cells from immune chickens into naïve chicks prior to infection can protect them from acute disease[4].
As a result there is clearly a benefit to be gained by those COVID-19 vaccines that have been shown to be eliciting a cell-mediated immune response in addition to an antibody response, since this is likely to improve both protective immunity and viral clearance.
In addition to conventional needle inoculation, some veterinary vaccines are given orally (IB, for example) or as an oral prime followed by an intramuscular boost (TGE for example), which would suggest that local mucosal immunity may also have a role to play in protection against coronavirus disease and that this could be a first line of defence against a respiratory infection such as COVID-19.
Furthermore, there have been concerns regarding the genetic stability of the SARS-CoV-2 virus, with a lot of work devoted to studying global strain variability. With this in mind it is interesting to note that strain variation has also presented challenges to effective control of IB by vaccination in poultry, but that this can be addressed by including multiple virus strains within a vaccine or by inoculating with one vaccine strain and then boosting with a different strain to broaden the host’s immune response.
Safety concerns
While the experience of successful coronavirus vaccine production within veterinary medicine bodes well for the possibility of developing vaccines for COVID-19, approaches to develop a vaccine against feline infectious peritonitis (FIP) have encountered a number of safety concerns.
The most cautionary results come from a recombinant vaccinia virus-encoded FIP virus spike protein approach, which resulted in early death following a virulent FIP challenge of immunised kittens. Further data[5] indicated that the ability of an FIP vaccine to protect cats against experimental challenge is dependent upon the strain and particularly the dose of challenge virus. Low-dose exposure resulted in protection of vaccinated cats, while higher-dose exposure resulted in virtually no protection and induced accelerated FIP in many cases as a result of antibody-dependent enhancement (ADE).
Acute respiratory disease in poultry. Live attenuated virus administered through drinking water, aerosol spray or oculo-nasally. • Bovine coronavirus
Causes neonatal calf diarrhoeal disease. Inactivated whole virus, given intramuscularly combined with other vaccines. • Canine coronavirus
Sudden vomiting, depression, diarrhoea, and dehydration or respiratory disease. Inactivated feline enteric coronavirus (FECV), antigenically similar to enteric CCV, administered with other vaccines by injection to young puppies followed by a second booster dose. • Feline infectious peritonitis
Various symptoms including fluctuating fever and death. Attenuated, temperature- sensitive strain via intranasal vaccination. • Porcine transmissible gastroenteritis
Acute diarrhoea and vomiting in young pigs. Live, attenuated whole virus, via intramuscular injection alone or combination of oral prime followed by an intramuscular boost.
A follow-up study using monoclonal antibodies identified the sites on the FIP spike protein which mediate the ADE following vaccination. Thus, it is important that those developing vaccines against COVID-19 should be aware of the potential risk of ADE induction leading to enhanced disease following vaccination and take care to address this in their safety studies. In the light of these observations regarding existing veterinary coronavirus vaccines, it is interesting to review where we are currently with our efforts to develop a vaccine against COVID-19.
As of the beginning of November 2020 the World Health Organization reported that there were 202 vaccines in development against the SARS-CoV-2 virus and that 47 of these potential candidates were already in human Phase 1, 2 or 3 clinical trials. What is even more remarkable is that the vaccines within these trials are using eight different vaccine technologies (inactivated whole virus, non-replicating viral vector, replicating viral vector, DNA delivery, messenger RNA delivery, self-amplifying RNA delivery, protein subunit and virus-like particle). Indeed, many of the more novel vaccine ‘platform’ technologies that are being employed to rapidly develop COVID-19 vaccines often find their first application within veterinary medicine where they already have a proven track record of safety and efficacy[6].
While the initial signs are encouraging[7,8], we now await the outcome of these critical human trials in terms of safety and efficacy data, although the latter will require a significant number of virus exposures to prove that a protective immune response has been produced – in a climate of diminishing disease within some countries.
We can see that research to find a vaccine against COVID-19 is making rapid progress and we can be assured that the prospects are good, since coronavirus vaccines have been successfully developed against a range of veterinary coronavirus diseases using differing technologies and immunisation strategies. These existing vaccines provide us with useful supportive data and the 'One Health' lessons from veterinary medicine should prove of some value in our hunt for a safe and efficacious vaccine against COVID-19.
Dr Michael James Francis CBiol FRSB has more than 40 years of experience in industrial vaccine R&D, and has helped develop a wide range of veterinary vaccines against viral, bacterial and parasitic diseases. He is currently the managing director of vaccine development consultancy BioVacc Consulting Ltd.
1) Salyer, S. J. et al. Prioritising zoonoses for global health capacity building. Emerg. Infect. Dis. 23(Suppl 1), S55 (2017).
2) Francis, M. J. Vaccination for One Health. Int. J. Vaccines Vaccin 4(5), p00090 (2017).
3) Crouch, C. F. et al. Serological, colostral and milk responses of cows vaccinated with a single dose of a combined vaccine against rotavirus, coronavirus and Esherichia coli F5(K99). Vet. Rec. 149(4), 105–108 (2001).
4) Collisson, E. W. et al. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp.Immunol. 24(2–3), 187–200 (2000).
5) Scott, F. W. et al. Evaluation of the safety and efficacy of Primucell-FIP vaccine. Feline Health Topics 7(3), 6–8 (1992).
6) Francis, M. J. Recent advances in vaccine technologies. Vet. Clin. North Am. Small Anim. Pract. 48(2), 231–241 (2018).
7) Folegatti, P. M. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet, published online 20 July. https://doi.org/10.1016/S0140-6736(20)31604-4 (2020).
8) Jackson, L. A. et al. An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. N. Engl. J. Med. DOI: 10.1056/NEJMoa2022483, 1–12 (2020).


