Sowing the seeds of plant science

The Biologist looks at how a series of DEFRA-sponsored studentships are helping to build resilience and capacity in the UK plant health sector
This year the RSB is again partnering with DEFRA to offer 12 plant health undergraduate studentships. The studentships are part of a suite of initiatives that the Society and DEFRA hope will build a diverse and resilient community of plant health scientists in the UK over the coming decade.
As part of the placements, undergraduates receive funding to join plant health professionals for 8-10 weeks over their summer holiday and work on a plant science research project. This year the projects available include detecting stress in raspberry plants, investigating pathogenic fungi in Scots pine trees, changing the behaviour of insect viral vectors and the use of ‘biostimulants’ to improve plant health.
With the latest round of studentships set to be announced this summer, The Biologist asked last year’s cohort of students to write about the importance of their experience and the research they contributed to.
Isla Redgwell Welch
Natural Sciences student at Durham University
Plants are the coolest. They have always been at the forefront of human history, as a source of food, medicine, culture, and more. And they are now at the forefront of one of the largest problems facing the modern world. How do we feed an ever-growing population, in a way that won’t cause further damage to a rapidly warming world?
I was recently awarded the incredible opportunity to explore these questions as part of the RSB’s PHUGS scheme at Lincoln University. Supervised by the wonderful Dr Sandra Varga and Dr Sheena Cotter, I undertook a project investigating the individual and synergistic effects of two types of fungi as a means of sustainable pest control against Pieris brassicae larvae.
Arbuscular mycorrhizal fungi (AMF) are soil-borne fungi which form mutualistic relationships with the roots of plants through the production of arbuscules – finely branched hyphae within root cells of the host. This helps plants take up nutrients and increases its capacity to cope with pest stress. Beauveria bassiana directly parasitises certain crop pests through production of secondary metabolite toxins which infect and kill its host. It has been hypothesised there may exist a synergistic relationship between these fungi, but little data exists concerning this interaction.
We first explored the effects of B. bassiana in petri dishes. There was no significant difference in the mass of Brassica oleracea leaves eaten by Pieris brassicae larvae when leaves were sprayed with B. bassiana compared to control leaves. In the field however, spraying Brassica oleracea plants with B. bassiana significantly increased above ground biomass through reducing herbivory, but the role of P. brassicae in this is uncertain.
Upon staining our brassica roots using a very ‘kitchen chemistry’ method of ink and vinegar (and KOH), none of the roots were found to be colonised with AMF. Additionally, there was no statistical difference identified between the control and supposedly AMF-inoculated groups. Brassicas are largely non-mycorrhizal plants which likely explains this finding and so a different model should be used in future.
 Pieris brassicae larvae
Pieris brassicae larvaeThe results of this project, though largely inconclusive, further support the idea that naturally occurring pesticides can be as effective as their synthetic counterparts without the oft-associated loss of biodiversity. Poor soil health is a large risk when non-selective chemical pesticides are overused, and a link between soil microbes like AMF and a reduction in pest damage could represent a very complete solution.
Personally, this project has also been invaluable in improving my confidence in using statistical test. It also gave me an insight into the intricacies and unpredictable nature of practical fieldwork – such as when several of our caterpillars were infected with parasitoid wasps and exploded into a pale wriggling mass of larvae. Fascinating but somewhat counterproductive.
Daisy Furrokh
Undergraduate biology student at the University of Bristol
Last year I undertook a research studentship on carrot family viruses at Fera Science. I was excited to find this studentship as I had developed a keen interest in plant disease and food security during my degree. Through the course of the studentship this grew into a fascination for viruses, not only their devastating effects on crop yield but also the strangely beautiful symptoms they can cause in plants.
We aimed to characterise two novel viruses, a potyvirus and a potexvirus, which had been isolated by high throughput sequencing of samples of Apiaceae spp. weeds which surrounded a carrot field. This was done by mechanically inoculating indicator plants to visualise their symptoms, as well as genome sequencing and bioinformatic analysis. The mechanical inoculation was challenging and required patience and persistence. Sequencing allowed a genome annotation to be constructed, showing the virus genome regions present, and confirming that the viruses were not aphid transmitted. This, however, raised the need for further research to explain how the viruses were transmitted.
This research is part of a larger project aiming to understand interactions between weed and crop hosts of viruses. This research is important – by understanding patterns of disease transmission, practices can be put in place to minimise yield loss and therefore ensure greater future food security. I found my studentship extremely fun, and it solidified my understanding and competence in molecular techniques which I was previously weary of. It was great spending so much time every day with scientists living my dream career. I now feel inspired to pursue a career in plant health research to help tackle the predicted future global food shortages.
 Daisy Furrokh (left) with a colleague on her PHUGS studentship
Daisy Furrokh (left) with a colleague on her PHUGS studentship Jasper Chippendale
Biochemistry student at the University of York
What attracted me to the idea of working in plant pathology was my interest in unknown organisms – those where we know little about physiology, their life, and fundamental cell biology. I have also always been fascinated by plants and how they survive in such a hostile world. My research project explored the disease caused by the fungal pathogen Fusarium graminearum in common wheat. As the crop is globally cultivated, the pathogen has spread alongside it, and causes a 20-50% reduction in yield and decreased quality due to accumulation of toxins.
My project focused specifically on resistance in a worldwide panel of wheat landraces, using a combination of transcriptome analysis, and assessment of a range of seedling based assays to effectively model adult plant resistance. The work I did found significant hub genes related to fungal resistance that were differentially expressed across the panel of landraces, showing potential for breeding to improve resistance. We met significant challenges in assessing resistance of landraces at the seedling stage using a range of techniques, often due to the different germination rates and efficiencies of the different seeds.
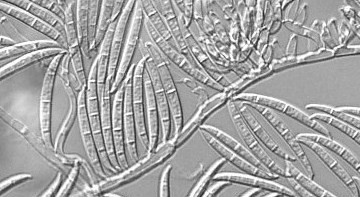 The fungal wheat pathogen Fusarium graminearum
The fungal wheat pathogen Fusarium graminearumPersonally I gained a lot from this experience both as a scientist and as a person. I have found a subject I am very interested in. From a research point of view I developed an appreciation for experimental design and the fact that not all experiments go to plan – troubleshooting is a fundamental part of working in the lab. My skill with R has improved a lot, and I became a lot more confident as I felt like a valued member of the lab, even if a less experienced one.
Lucia Nikolaeva-Reynolds
Integrated masters student at the University of Bristol
Most of us know the harm that the overuse of pesticides causes to planetary and human health. Our over-reliance on pesticides in agriculture contributes to biodiversity loss through their effects on non-target organisms and the evolution of resistant pests. Pest management must therefore be undertaken in a more sustainable way.
Supervised by Dr József Vuts and Dr Gareth Thomas in the Protecting Crops and the Environment (PCE) department at Rothamsted, my project explored sustainable management of Sitona lineatus, known as the bean weevil or pea leaf weevil. The larvae of this common pest of plants in the bean family feed on the nodules associated with the leguminous roots, causing significant yield losses in temperate regions. Nodules are formed by the Rhizobium bacteria that have a symbiotic relationship with the plant. It was hypothesized that the root-feeding herbivores use volatile compounds released by the bean root nodules – or rhizobia within the nodules – to find and identify the plant host as a food source.
 The pea leaf weavil Sitona lineatus
The pea leaf weavil Sitona lineatusThe essays forming this article were written by PHUGS students 2022.


